All about the West Wing and clinical drug trials
The West Wing Clinical Research Centre is a dedicated space in our Sutton hospital, for complex clinical trials.
About the West Wing
The centre enables the rapid and effective translation of scientific findings regarding the genetic and molecular basis of cancer into improved targeted therapies. These trials test new cancer treatments, methods of dealing with side effects and how a new treatment compares to an existing one.
The centre opened in 2014 and cost £2.6 million - this was entirely funded by gifts in Wills, generously left by The Royal Marsden Cancer Charity supporters.

About clinical drug trials
Clinical trials are divided into different stages, called phases. Early phase trials may look at whether a drug is safe, or the side effects it causes. Later phase trials test whether a new treatment is better than existing treatments.
The Oak Drug Development Unit is the second biggest unit for Phase 1 drug trials in the UK. Phase 1 trials help to determine the dose of the drug, frequency and what tumour types respond best.
Abiraterone, a ground-breaking drug for treating patients with prostate cancer, was tested at the Oak Drug Development Unit. Adding Abiraterone to hormone therapy at the start of treatment for prostate cancer was found to improve survival rates by 37 per cent.
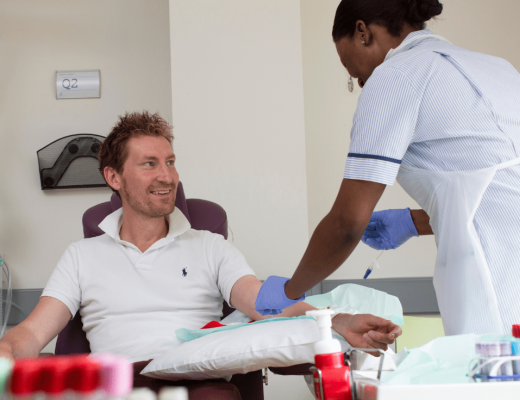
More recently, there has been a focus on cellular therapy trials. This involves human cells being harvested from the patient, modifying the cells then returning them back to the patient. This can then repair damaged tissue and/or cells. Thomas, pictured left, benefited from a cellular therapy trial.
Due to the success of this treatment, The Royal Marsden became one of the few accredited cellular therapy sites in the UK.
Clinical trials at the West Wing are helping to develop more efficient and effective treatment methods.
With your support we can continue to invest in research and treatment for cancer.