‘My whole world changed when I was diagnosed with stage 4 melanoma, but immunotherapy has saved my life'.
Zoe is one of many patients who has benefitted from a new immunotherapy treatment, Lifileucel. Now, latest research at The Royal Marsden shows it can improve survival rates for those with advanced melanoma.

In June, Dr Andrew Furness, Consultant Medical Oncologist and Solid Tumour Cellular Therapy Lead at The Royal Marsden, shared promising findings from the C-144-01 clinical trial at the American Society of Clinical Oncology (ASCO) annual conference which showed exciting advancements in the treatment of advanced melanoma.
The C-144-01 immunotherapy trial, an international trial led by The Royal Marsden, explored the use of Lifileucel as a one-time treatment for patients who have not responded to other forms of treatment.
Just 15 years ago, advanced melanoma was considered untreatable. In fact, before immunotherapy – a type of treatment which harnesses the power of a patient’s own immune system to fight cancer cells - only 5% of patients survived beyond five years of diagnosis.
The results presented at ASCO showed that the use of Lifileucel had a significant impact on the survival rate of many patients on the trial, and a clear reduction in tumour size for the majority of them:
Nearly one in five patients (19.7%) were still alive five years after treatment, and it was shown that tumours had shrunk in 79.3% of patients overall.
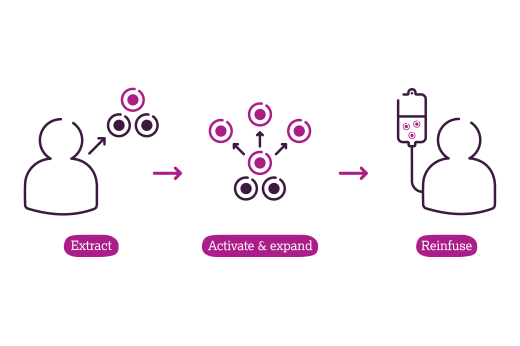
What is Lifileucel and how does it work?
Lifileucel is a type of T-cell immunotherapy known as TIL therapy. During TIL therapy, part of the tumour is surgically removed in order to harvest T cells that are naturally capable of infiltrating the cancer. These are then activated and expanded (grown in large numbers) in a lab before being reinfused into the patient. This gives the patient more T-cells that are better able to recognise and attack cancer cells.
TIL therapy is the first ever cellular therapy approved by the U.S. Food and Drug Administration (FDA) for use on solid tumours.
Cellular therapies like Lifileucel could be the future of cancer treatment.
TIL therapy may transform outcomes for people with advanced melanoma
Dr Andrew Furness is part-funded by The Royal Marsden Cancer Charity. Thanks to our supporters, we are able to provide funding towards pioneering research taking place at the hospital which benefits cancer patients in the UK and around the world.
Dr Andrew Furness said: “While current forms of immunotherapy have revolutionised the treatment of cancer in recent years, overall these benefit a minority rather than majority of treated patients.
“The C-144-01 study demonstrates that even when other immunotherapies have failed, the immune system can be harnessed in a different way to reliably control a patient’s cancer. Results from this trial have shown that TIL therapy may change the outlook for people with advanced melanoma.
“We’re continuing our research into the use of TIL therapy, as well as other forms of cellular therapy, across a broader spectrum of cancers including advanced lung, liver, ovary, skin and testicular subtypes as well as soft tissue sarcoma.”
Lifileucel changed Zoe’s life after her stage 4 skin cancer diagnosis

47-year-old Zoe was diagnosed with stage 4 metastatic melanoma in 2023, and was referred to Dr Furness at The Royal Marsden. She was placed on the IOV-COM-202 clinical trial exploring the use of Lifileucel as a first-line treatment, meaning she had never tried any other treatment previously.
Receiving the results from her first scan after the Lifileucel treatment was a nerve-wracking experience for Zoe.
“When it came to my six-week scan post treatment, it was a really big moment for us all to see if it had worked. I was freaking out.
“Dr Furness held up my scans, the one before treatment where you can clearly see the tumour and the one taken six weeks later – you couldn’t see anything. There was nothing on the second scan. The treatment had worked – we were all absolutely over the moon.
I cried as I was so happy, I just didn’t know what to say.

Now, Zoe’s regular check-up scans have been clear for 18 months, and her body is showing no signs of cancer.
Zoe said: "My type of cancer is considered incurable, but there is currently no evidence of cancer in my body - it’s amazing. It’s been a long journey.
“Before coming to The Royal Marsden I was told I would probably die, so to now be stable on this trial – it’s been such a rollercoaster. Some days it doesn’t feel real.
“Being alive is the best present ever."
“It’s incredible that this treatment, which had such a huge impact on me, is now helping patients who haven’t responded to other methods of treatment. I always hoped that my involvement in research would help lots of others, and it’s amazing to know that the success of my trial isn’t just for me – it's for everyone.
“What The Royal Marsden and this trial has done for me is amazing. It’s saved my life and is enabling me to continue making memories with my family.”
Solid tumour cellular therapy research at The Royal Marsden is funded by supporters of The Royal Marsden Cancer Charity including a significant donation from Nationwide Building Society, as part of their Fairer Futures social impact strategy. The C-144-01 clinical trial was funded by Iovance Biotherapeutics. Funding was also contributed from The National Institute for Health and Care Research (NIHR) Biomedical Research Centre at The Royal Marsden NHS Foundation Trust and The Institute of Cancer Research, London.
Help us support pioneering treatments
The Royal Marsden changes the lives of countless people like Zoe every year. Thanks to your support, we can continue to make life-saving research breakthroughs and develop new and better treatments for cancer.
Find out more about ways you can support us today.
Read more stories in our blog


